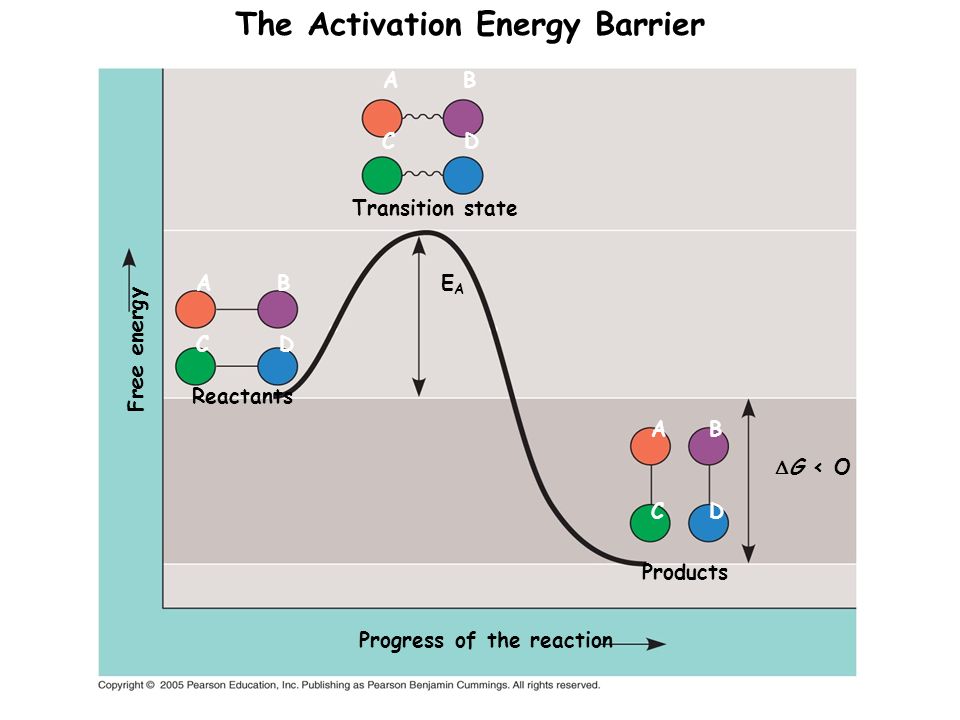
Kinetics of the base hydrolysis of 6-nitro-2H-chromen-2-one (NC) and 6-nitro-2H-chromen-2-one-3-carboxylic acid (NCC) in water-methanol and water-acetone mixtures was studied at temperature range from 283 to 313 K. The activation parameters of the reactions were evaluated and discussed. The change in the activation barrier of the investigated compounds from water to water-methanol and water-acetone mixtures were estimated from the kinetic data. ...
Read more


.jpg)

.jpg)


.jpg)