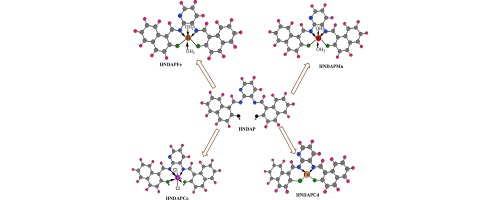
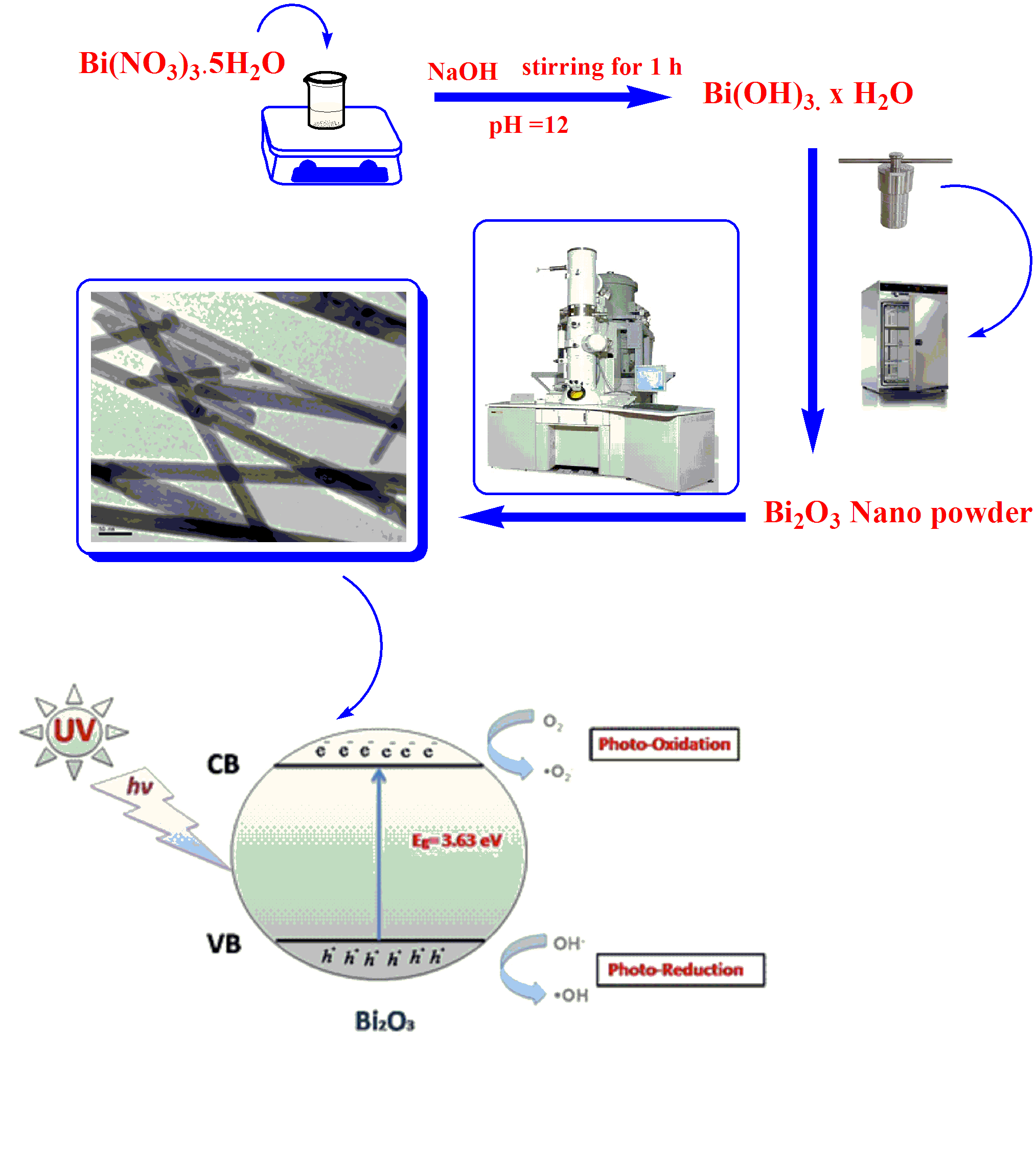
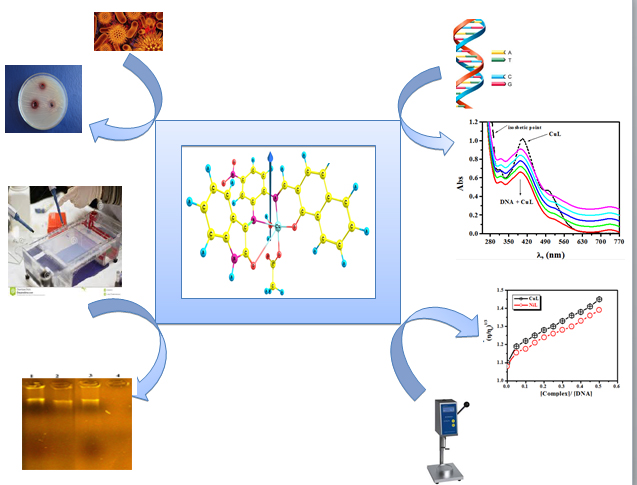
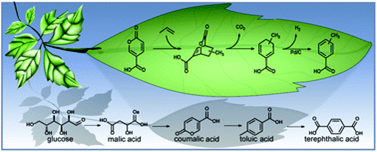
This study highlights synthesis and characterization of a tetradentate ONNO Schiff base ligand namely (1, 1′- (pyridine-2, 3-dimethyliminomethyl) naphthalene-2, 2′-diol) and hereafter denotes as “HNDAP″ and selected metal complexes including Mn(II), Fe(II), Co(II) and Cd(II) as a central metal. HNDAP was synthesized from 1:2 M ratio condensation of 2, 3-diaminopyridine and 2- hydroxy-1-naphthaldhyde, respectively. The stoichiometric ratios of the prepared ...
Read more