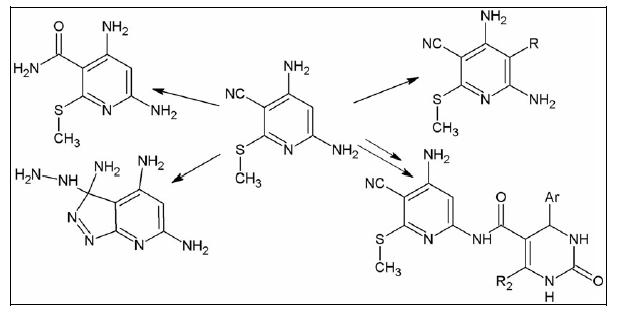
Treatment of 4,6-diamino-3-cyano-2-methylthiopyridine (1) with aqueous KOH or hydrazine hydrate afforded the corresponding nicotinamide 2 and pyrazolo[3,4-b]pyridine 3, respectively. Reaction of compound 1 with bromine, sulfuryl chloride, formaldehyde, or aromatic diazonium salts gave 5- bromopyridine 4, 5-chloropyridine 5, dipyridylmethane 6, and azo dyes 7–10, respectively. Compound 1 reacted with diketones to yield the corresponding butenylamino derivative 11 and amides 12–15, ...
Read more


.jpg)
.jpg)

![Crystal structure of N-[4-amino-5-cyano-6-(methylsulfanyl)pyridin-2-yl]-2-chloroacetamide](https://staffsites.sohag-univ.edu.eg/uploads/1502/1540114726images (5).jpg)
.jpg)
![Crystal structure of N-[4-amino-5-cyano-6-(methylsulfanyl)pyridin-2-yl]acetamide-hemihydrates](https://staffsites.sohag-univ.edu.eg/uploads/1502/1540296261images (2).jpg)
![Ethyl 2-[({[4-amino-5-cyano-6-(methylsulfanyl)pyridin-2-yl]carbamoyl}methyl) sulfanyl]acetate monohydrate](https://staffsites.sohag-univ.edu.eg/uploads/1502/1538412535images 22223.jpg)
![3,4,6-Triamino-N-phenylthieno[2,3-b]pyridine-2-carboxamide](https://staffsites.sohag-univ.edu.eg/uploads/1502/1538412619images 22223.jpg)
.jpg)