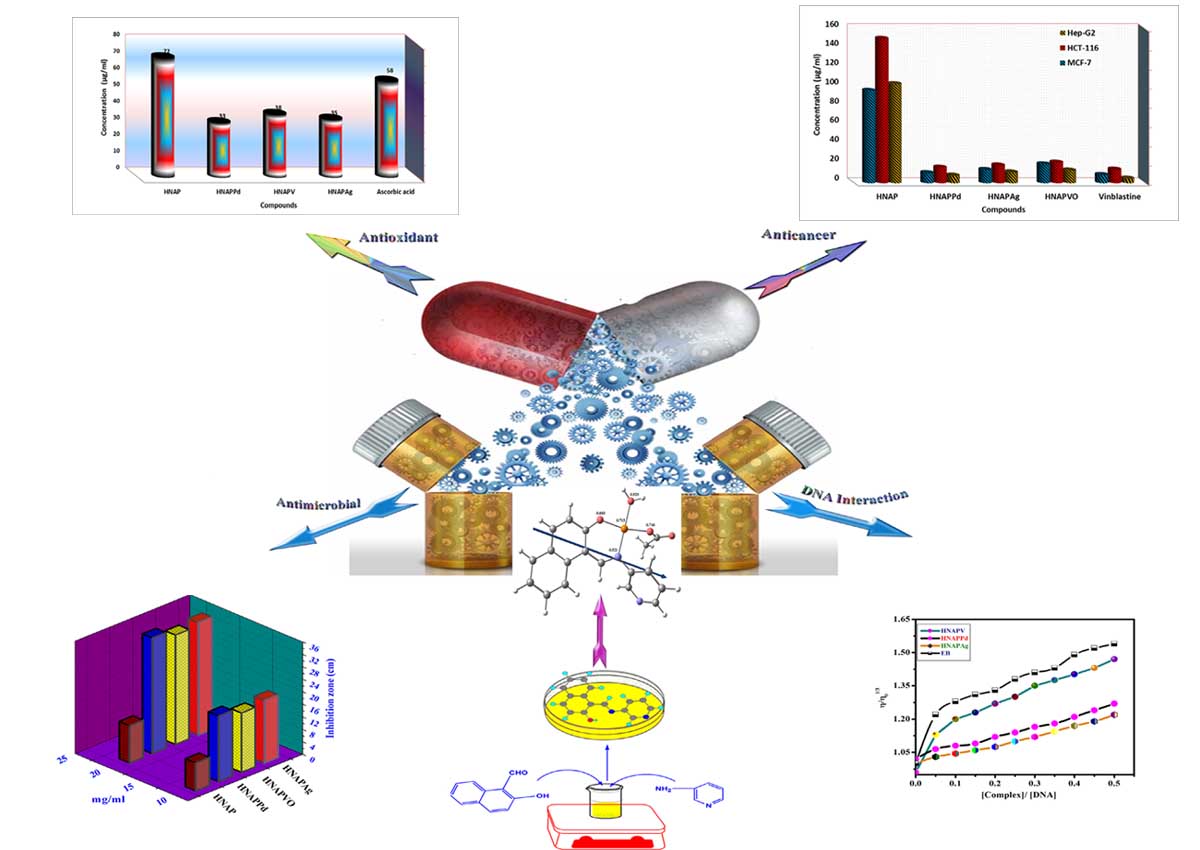
A novel azomethine ligand (HNAP) [HNAP = 1-(Pyridin-3-yliminomethyl)-naphthalen-2-ol] and its Ag(I), Pd(II) and VO(II) chelates have been synthesized and structurally inspected using a wide range of spectroscopic and analytical tools, including infra-red (IR), ultraviolet-visible (UV-Vis) and 1H NMR spectroscopy techniques, CHN analysis, molar conductance, magnetic susceptibility, and thermogravimetric analysis. The molar conductance measurements reveal that the chelates are non-electrolytes. The thermal behavior of the investigated metal chelates shows that the hydrated, coordinated water molecules and the anions are removed in successive steps followed immediately by decomposition of the ligand in the subsequent steps. The activation thermodynamic parameters are calculated from the TG curves and discussed. Complexes formation study via continuous variation m molar ratio has been investigated, and results were consistent to those found in the solid complexes with a ratio of (M:L) as (1:1) or 1:2 (M:L) molar ratio for all the monolithic and bi-valent metal complexes with square planar for Pd(II), and Ag(I) cations while, square pyramidal geometry for VO(II) cation. DFT calculations for the titled different metal-chelates have been studied and showed a good correlation with the experimental data. The prepared compounds had been checked In vitro towards numerous sorts of plant pathogenic fungi and bacteria to evaluate their antimicrobial properties and compared with some known antibiotics. Significantly, all the complexes show excellent antimicrobial activity against various strains of bacteria and fungi, including both Gram-negative and Gram-positive bacteria. Besides, the complexes exhibited high cytotoxicity against various carcinoma cell lines, including HCT-116, MCF-7, and HepG-2. Moreover, the effect of the new synthesized compounds as antioxidants was determined by reduction of 1,1-diphenyl-2-picryl hydrazyl (DPPH) and compared with that of Vitamin C . Furthermore, the binding interactions of the complexes with CT-DNA were explored using UV-Vis spectroscopy, viscosity and gel electrophoreses measurements. They cooperatively bind to DNA possibly through intercalations. The binding ability of the complexes was shown as HNAPAg > HNAPPd > HNAPVO complex.