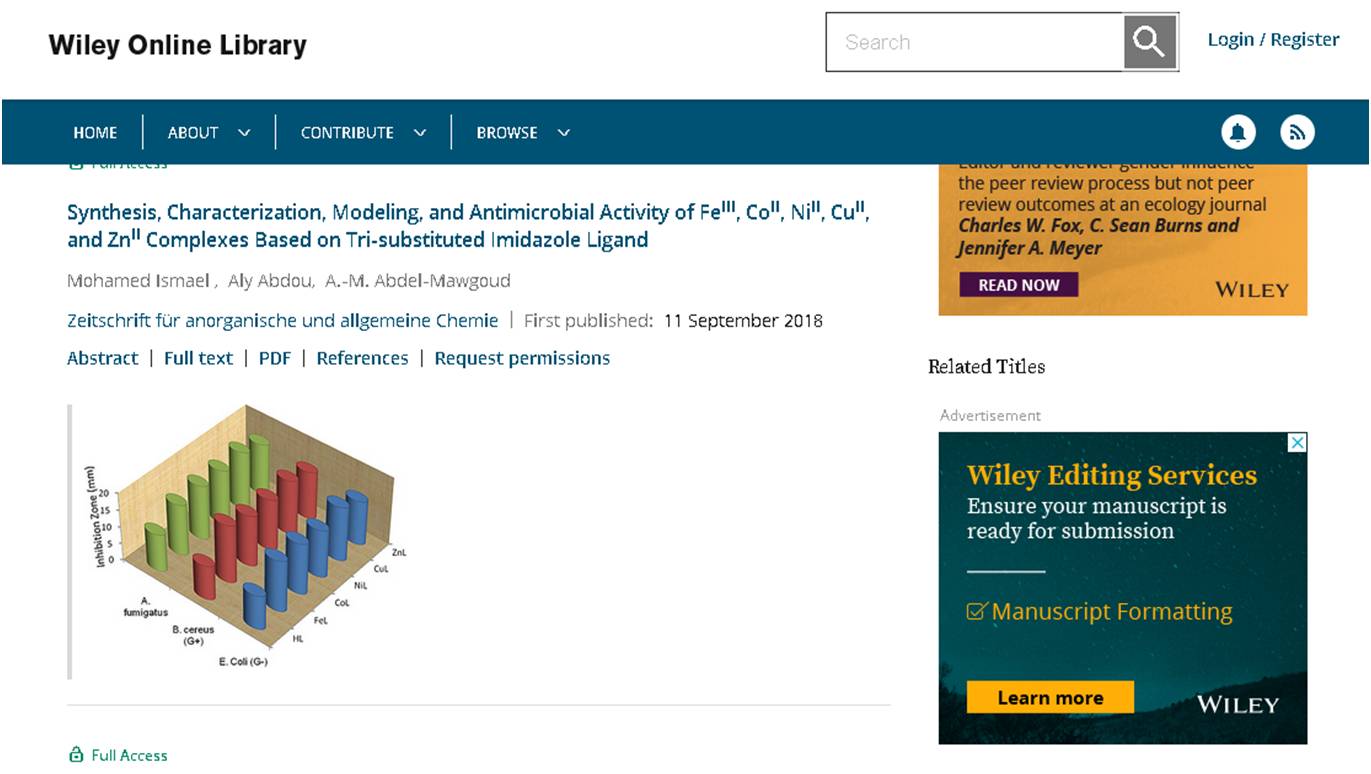
The reaction of the synthesized 2‐(4,5‐diphenyl‐1H‐imidazol‐2‐yl) phenol, (HL) ligand with FeIII, CoII, NiII, CuII, and ZnII ions at room temperature resulted in the formation of the five complexes; [Fe(L)2(H2O)(Cl)]·2H2O and [M(L)2)]·nH2O [M = Co, Zn (n = 2), Ni, Cu (n = 1)]. The ligand and its complexes were characterized based on elemental analyses, spectral, magnetic and molar conductance measurements. The molecular and electronic structure of the ligand and its complexes was optimized theoretically and the quantum chemical parameters were calculated. The results revealed an interesting geometrical variation; octahedral for FeL, seesaw for CoL, distorted square planar for NiL and CuL, as well a tetrahedral arrangement for ZnL. The HL ligand and its metal complexes were screened against the growth of pathogenic bacteria [Escherichia coli (G–) and Bacillus cereus (G+)] and fungi (Aspergillus fumigatus) in terms of the minimum inhibitory concentration (MIC). The metal complexes showed high enhancement as antimicrobial candidates with lower MIC values compared with their parent ligand. Structure activity relationship formula was quantified by correlating the experimental MIC values with theoretical electronic chemical properties. Molecular docking of the complexes against CYP51B protein of A. fumigatus, the target enzyme for the antimicrobial reagents, was achieved to find the best orientation of the substrate, which would form a stable complex with overall minimum energy. The docking results enhanced the activity of the imidazole‐based complexes as promising antimicrobial candidates.