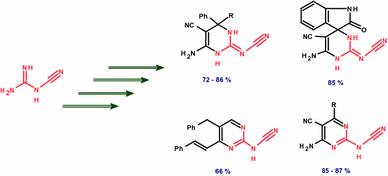
A novel series of 4-aryl-2-cyanoimino-3,4-dihydro-1H-pyrimidines (aryl-CIDHPMs) was synthesized using a new method via the one-pot three-component reaction of cyanoguanidine with malononitrile and aromatic aldehydes using sodium methoxide as catalyst. These new aryl-CIDHPMs were also prepared by a classical route via reaction of cyanoguanidine with the corresponding arylidenemalononitriles under our conditions. In the same manner, the reaction of cyanoguanidine with cyclohexylidenemalononitrile and/or isatinylidenemalononitrile afforded new spiro-pyrimidines 9 and 10, respectively. A new series of polyfunctionalized 2-cyanoaminopyrimidines was obtained from the reaction of cyanoguanidine with cinnamaldehyde and/or ethoxyalkylenemalononitriles.