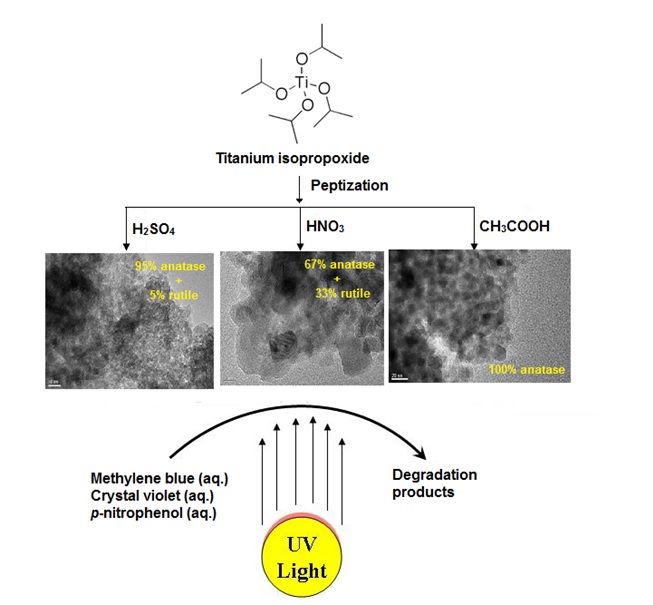
TiO2 nanoparticles were synthesized from titanium isopropoxide by a simple peptization method using sulfuric, nitric, and acetic acids. The effect of peptizing acid on physicochemical and photocatalytic properties of TiO2 powders was studied. The structural properties of synthesized TiO2 powders were analyzed by using XRD, TEM, N2-physisorption, Raman, DR UV-vis, FTIR, and X-ray photoelectron spectroscopy techniques. The characterization results showed that acetic acid peptization facilitated the formation of pure anatase phase after thermal treatment at 500 °C; in contrast, nitric acid peptization led to a major rutile phase formation (67%). Interestingly, the sample peptized using sulfuric acid yielded 95% anatase and 5% rutile phases. The photocatalytic activity of synthesized TiO2 nanoparticles was evaluated for degradation of selected organic dyes (crystal violet, methylene blue, and p-nitrophenol) in aqueous solution. The results confirmed that the TiO2 sample peptized using nitric acid (with rutile and anatase phases in 3:1 ratio) offered the highest activity for degradation of organic dyes, although, TO2 samples peptized using sulfuric acid and acetic acid possessed smaller particle size, higher band gap energy, andhigh surface area. Intrstingly, TiO2 sample peptized with nitric acid possessed relatively high theoretical potocurrent density (0.545 mAcm−2) and pore diameter (150 Å), which are responsible for high electron-holeseparation efficiency and diffusion and mass transportation of organic reactants during the photochemicaldegradation process. The superior activity of TiO2 sample peptized with nitric acid is due to the effective transferof photogenerated electrons between rutile and anatase phases.