This research paper describes the preparation, characterization and the hydrogen peroxide decomposition
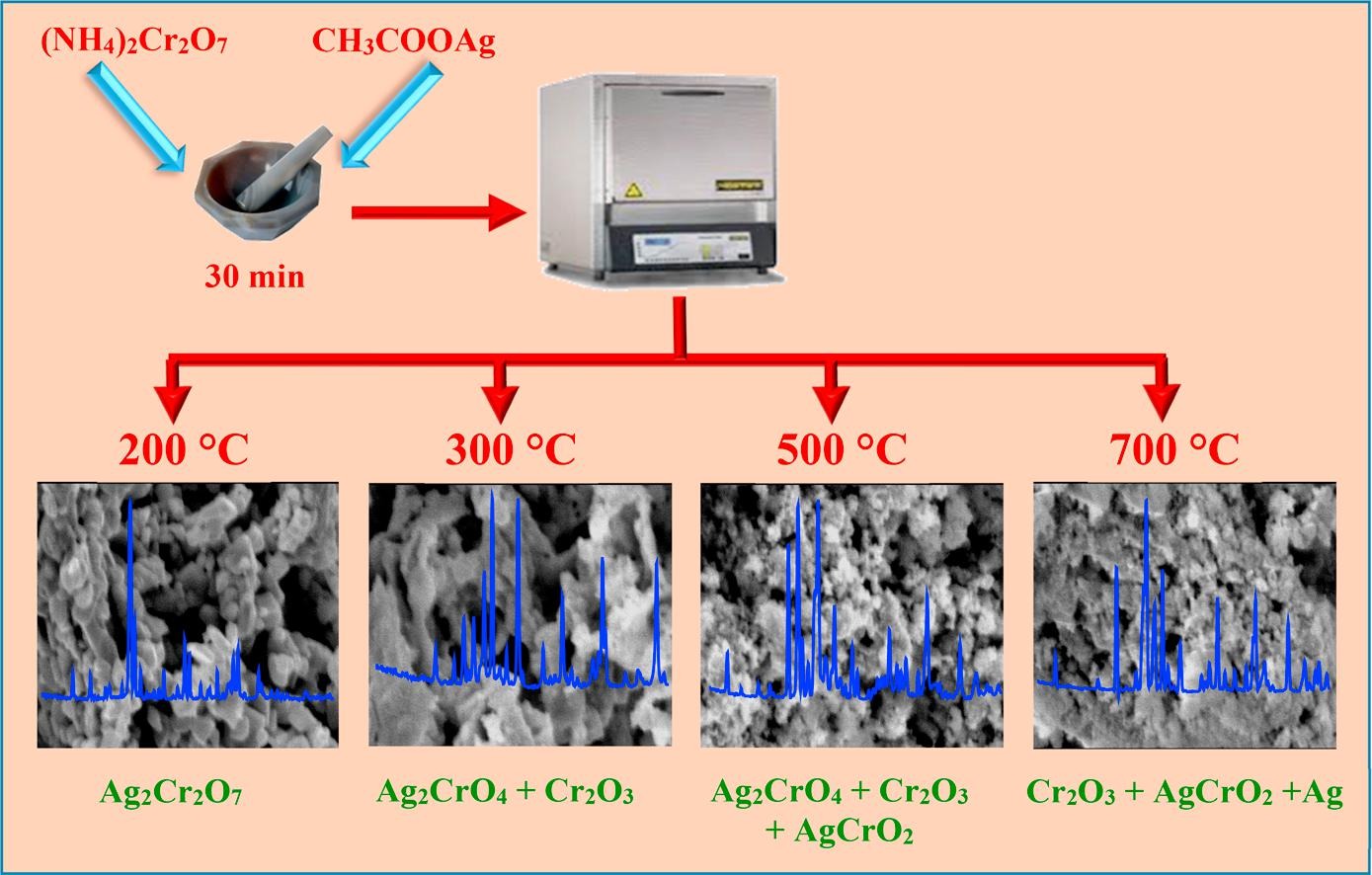
activity of a series of nano-crystalline Ag-Cr-O catalysts obtained by calcining a parent mixture of silver aetate and ammonium dichromate. This parent has been calcined for 1 h, in air, at the temperature range of200–700 °C. The characterization of the nano-crystalline Ag-Cr-O catalysts was performed using a series ofphysico-chemical analyses including TGA-DTA, XRD, FTIR, SEM, TEM, and XPS. The results revealed thatheat treatment brought about a significant-phase formation, Ag2Cr2O7 is formed at 200 °C and a mixture ofAg2CrO4 and Cr2O3 are formed over the calcination temperature range of 300–500 °C. AgCrO2 stats to form at500 °C and becomes a major phase at the range 600–700 °C with a noticeable segregation of metallic silver.The activity of the different nano-crystalline Ag-Cr-O catalysts was tested for hydrogen peroxide decompositionat the temperature range 35–50 °C. The kinetic data showed a direct dependence on the calcinationtemperature and it was correlated with the formed surface redox-couples.