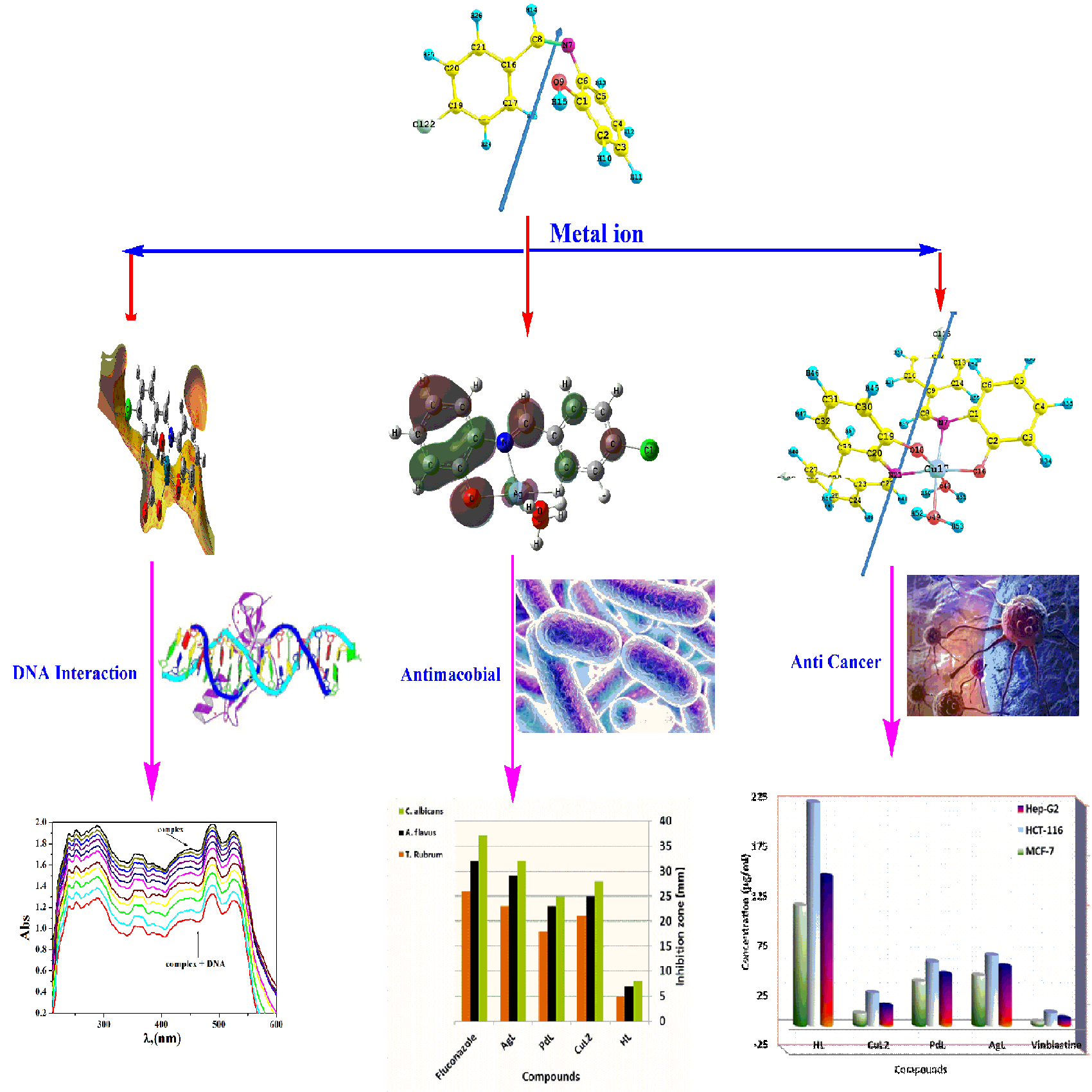
Three new Cu(II), Pd(II) and Ag(I) complexes with bi-dentate Schiff base ligand "HL" namely 2-[(4-Chloro-benzylidene)-amino]-phenol were synthesized. The stoichiometric ratios and physicochemical properties of these complexes were determined using elemental analyses (C, H, and N), magnetic measurements, IR, UV-Vis spectra, molar conductivity and thermal analyses. The results revealed that the metal ions coordinated with ligand HL through azomethine nitrogen and phenolic oxygen atoms. AgL and PdL complexes are present in a 1:1 molar ratio with square planar and tetrahedral geometry, respectively while CuL2 complex is present in a 1:2 molar ratio with octahedral (Oh) geometry. The electronic structure and nonlinear optical parameters (NLO) of the titled ligand HL and the studied 1:1 and 1:2 complexes were investigated theoretically at the DFT-B3LYP/6-311G** level of theory. The compounds were screened against various strains of bacteria and fungi. They displayed good results of inhibition against the studied pathogenic microorganism. The absorption spectroscopic, viscosity and gel electrophoresis measurements were used for studying the interaction of the investigated complexes with calf thymus DNA (CT-DNA). The studied complexes showed a good interaction with CT-DNA via intercalation and groove modes. Moreover, molecular docking of these complexes was studied to understand the drug–DNA interactions and calculate the potential binding mode and energy. Furthermore, the anticancer effects of HL and its complexes, on selected human carcinoma cell lines, were determined. The cytotoxic results showed that the prepared complexes are more potent than its Schiff base ligand.