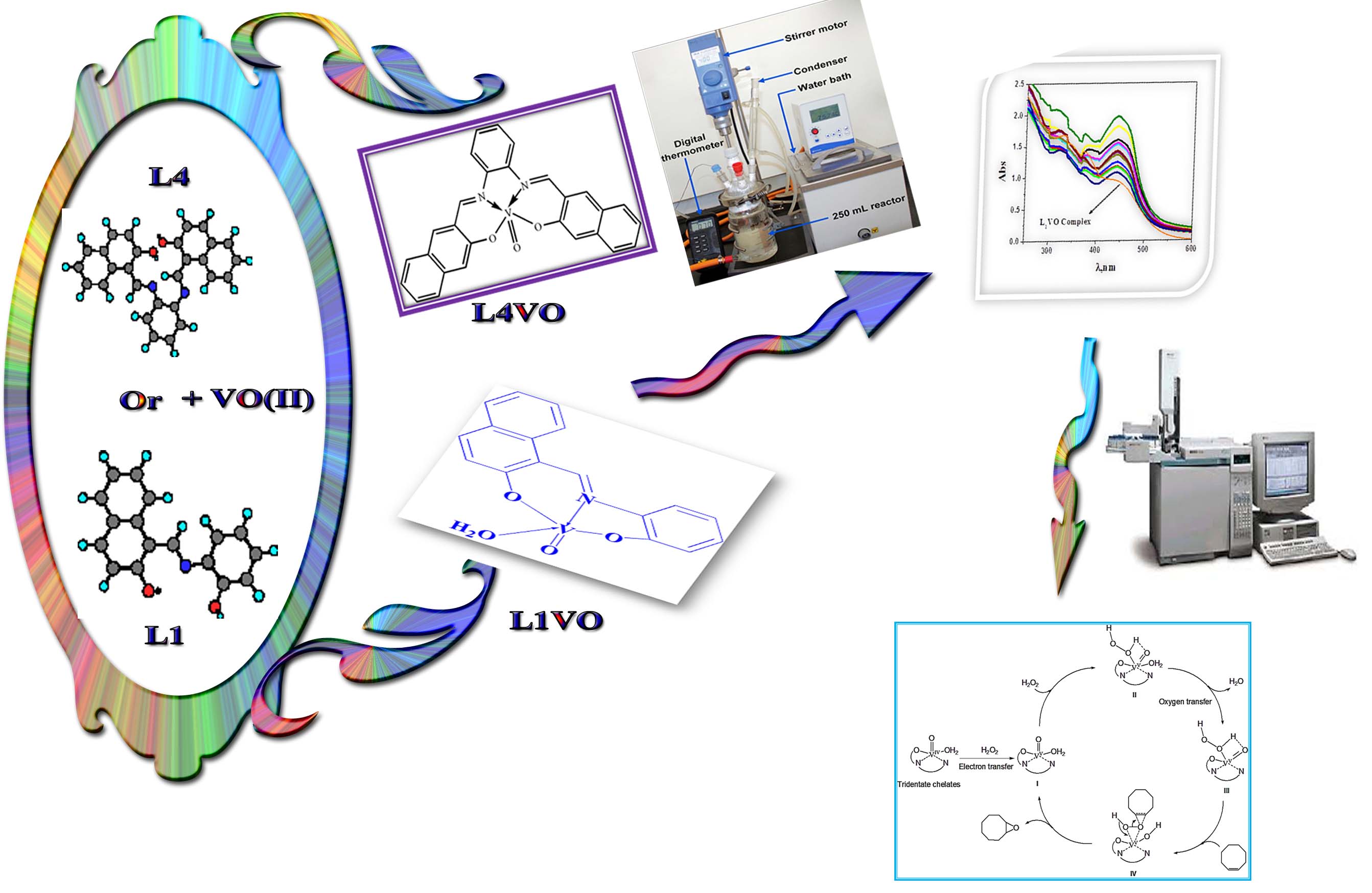
High yield of VO(II) chelates incorporating new imine ligands were obtained by the condensation reaction between 2-hydroxynaphthaldehyde or 3-ethoxysalicylaldehyde and o-phenylenediamine s in 1:1 molar ratio. All the complexes are non– hygroscopic in nature and stable to the atmosphere. The synthesized ligands and the isolated imine chelates have been characterized various physico-chemical techniques. The results obtained from these studies and their interpretation used to solve the structure of the prepared chelates. The results react with vanadyl acetylacetonate in 1:1 molar ratio to give distorted and square pyramidal geometry for tri- and tetra-dentate imine chelates, respectively. Moreover, the catalytic potential of the prepared VO(II) imine chelates has been tested for the epoxidation of cyclooctene using H2O2 as the terminal oxidant. Furthermore, the effects of various parameters of the molar ratio of catalyst to substrate, time, temperature and solvent have been studied. The catalytic processes are quantitative and highly selective with tr-dentate imine VO(II) complexes affording excellent yields of epoxide product. The optimal solvent and temperature for the cyclooctene epoxidation catalyzed by the prepared imine chelates is acetonitrile at 90 °C. The increase of the catalyst molar ratio reduced the selectivity of the epoxidation product with H2O2.