New series of Fe(II) complexes and Schiff base amino acids have been designed and synthesized
from the bioactive ligands by condensation of 5-bromo-2-hydroxybenzaldehyde and aamino
acids (L-alanine (ala), L-phenylalanine (phala), L-aspartic acid (aspa), L-histidine (his) and
L-arginine (arg)). Elemental analyses, infrared, ultraviolet–visible spectra, as well as conductivity
and magnetic susceptibility measurements are used to elucidate the structure of the newly prepared
iron(II) complexes. Moreover, the stoichiometry and the stability constants of the prepared complexes
have been determined spectrophotometrically. The results suggest that the prepared Schiff
base amino acid ligands behave as dibasic tridentate ONO ligands and bind to Fe(II) in octahedral
geometry according to the general formula [Fe(bs:aa)2].nH2O. The DNA interaction of these complexes
was tested at pH = 7.2, by using electronic absorption spectra and viscosity measurements.
The experimental results indicated that the investigated complexes could bind to DNA via
intercalative mode and showed a different DNA binding activity according to the sequence:
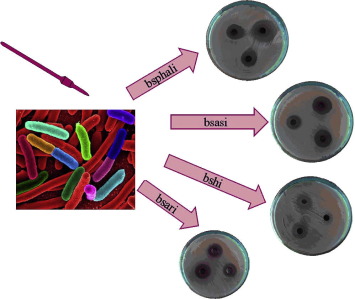
bsari > bshi> bsali > bsasi > bsphali. Moreover, the prepared compounds are tested for their
in vitro antibacterial activity against three types of bacteria, Escherichia coli, Pseudomonas aeruginosa
and Bacillus cereus. The results show that the metal complexes are more reactive with respect to their
corresponding Schiff base ligands.