The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders).
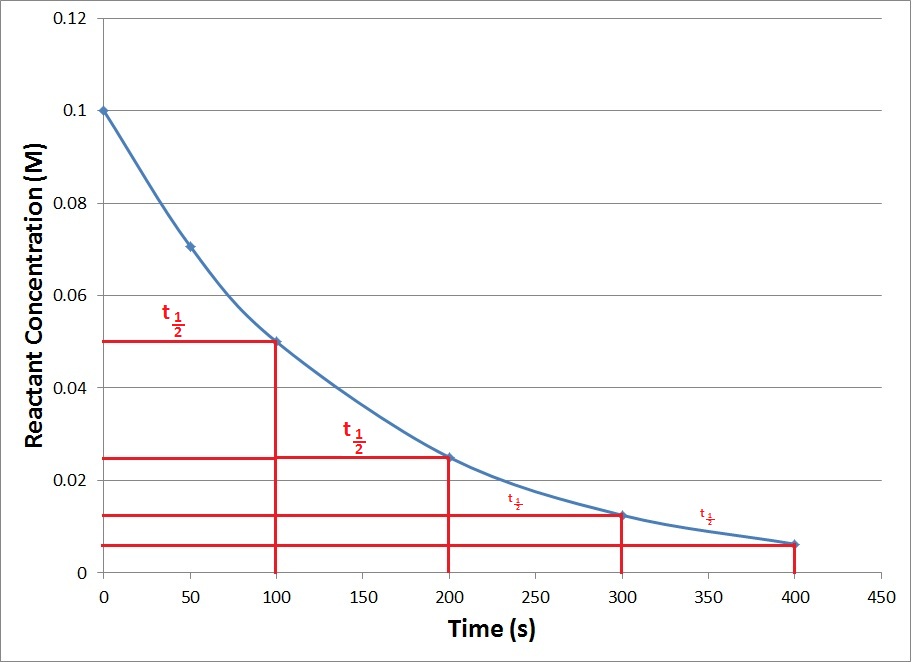
A first order reaction depends on the concentration of only one reactant (a unimolecular reaction)
In 1st order reaction:
What the relation between the initial concentration of the reactant and “the rate constant and half life time ”