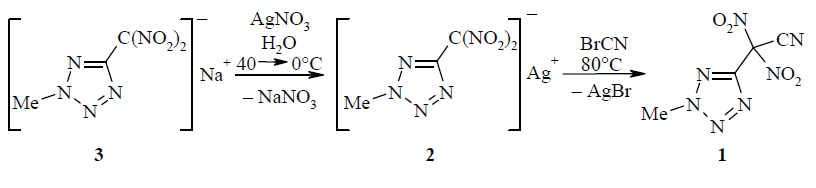
The reaction of the silver salt of (2-methyltetrazol-5-yl)dinitromethane with cyanogen bromide led to the formation of 2-(2-methyltetrazol-5-yl)-2,2-dinitroacetonitrile. The nitrile group of the latter was capable of entering into 1,3-dipolar cycloaddition with diazomethane, giving a mixture of isomeric 2-methyl-5-[(N-methyl-1,2,3-triazol-4-yl)dinitromethyl]tetrazoles that were separated by column chromatography. Upon treatment with an alcohol solution of potassium hydroxide, denitration of the cycloaddition products occurred and was accompanied by salt formation, leading to the potassium salts of 2-methyl-5-[(N-methyl-1,2,3-triazol-4-yl)(aci-nitro)methyl]tetrazoles.