Purpose
Acid ceramidase (AC) occupies an important place in the control of cancer cell proliferation. We tested the influence of AC inhibition on the effects of PSC 833, a P-glycoprotein antagonist with potent ceramide-generating capacity, to determine whether AC could be a therapeutic target in pancreatic cancer.
Methods
Ceramide metabolism was followed using 3H-palmitate, and molecular species were determined by mass spectroscopy. Apoptosis was measured by DNA fragmentation, autophagy by acridine orange staining, and cell cycle was assessed by flow cytometry and RB phosphorylation. AC was measured in intact cells using fluorescent substrate.
Results
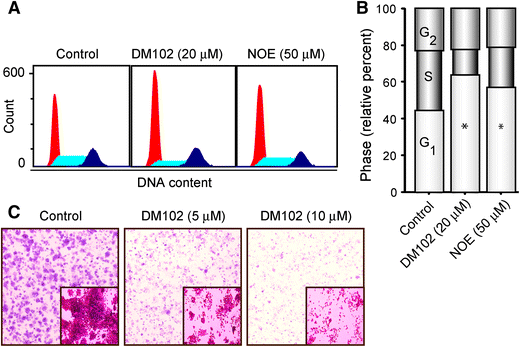
Exposure of human PANC-1 or MIA-PaCa-2 cells to PSC 833 promoted increases in de novo (dihydro)ceramides, (dihydro)glucosylceramides, and (dihydro)sphingomyelins, demarking ceramide generation and robust metabolism. Despite the multifold increases in (dihydro)ceramide levels, cells were refractory to PSC 833. However, PSC 833 produced a dose-dependent decrease in DNA synthesis and dose- and time-dependent decreases in RB phosphorylation, consistent with cell cycle arrest as demonstrated at G1. Cytostatic effects of PSC 833 were converted to cytotoxic end-point by acid ceramidase inhibition. Cytotoxicity was accompanied by formation of acridine orange-stained acidic vesicles and an increase in LC3 expression, indicative of autophagic response. Cell death was not reversed by preexposure to myriocin, which blocks PSC 833-induced ceramide generation.
Conclusion
Although the role of ceramide in end-point cytotoxicity is unclear, our results suggest that acid ceramidase is a viable target in pancreatic cancer. We propose that AC inhibition will be effective in concert with other anticancer therapies.