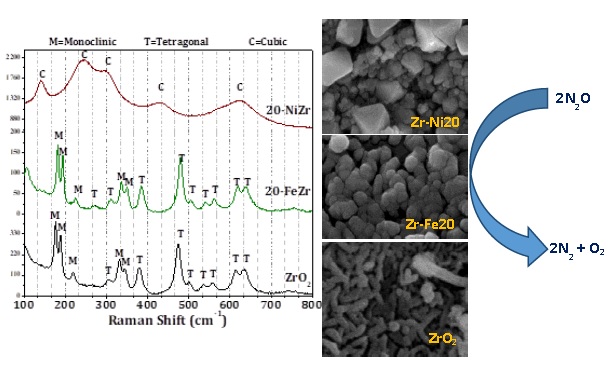
Mesoporous Fe2O3-ZrO2 and NiO-ZrO2 nanocomposites with 10 and 20 wt% of Fe2O3 and NiO composition were prepared by modified sol-gel method. The physico-chemical characteristics of calcined nanocomposites were evaluated utilizing different techniques such as X-ray diffraction, Raman spectroscopy, scanning electron microscopy, transmission electron spectroscopy, N2-physisorption, H2-temperature programed reduction, Fourier transformed infrared spectroscopy after pyridine adsorption and X-ray photoelectron spectroscopy. The N2O decomposition activity of calcined nanocomposite catalysts was evaluated using fixed bed reactor at different reaction temperatures. Bare mesoporous ZrO2 without any reducible oxide exhibited 30% N2O conversion at reaction temperature of 550oC. Fe2O3-ZrO2 nanocomposites with 10 wt% Fe2O3 offered enhanced N2O conversion (»95%) and stability for N2O decomposition at 550oC. Interestingly, NiO-ZrO2 nanocomposite with 20 wt% NiO exhibited lowest N2O conversion among the synthesized nanocomposites. The variations in N2O decomposition activity of Fe2O3-ZrO2 and NiO-ZrO2 nanocomposites could be expounded on the basis of catalyst characterization results. It was observed that presence of reducible M-O-Zr (M=metal) species along with crystalline tetragonal ZrO2 phase is essential to obtain better N2O decomposition. A complete ZrO2 phase transformation to cubic and large NiO particle size were responsible for the poor catalytic activity for NiO-ZrO2 nanocomposite with 20 wt% NiO. The durability of Fe2O3-ZrO2 and NiO-ZrO2 nanocomposites was tested for about 120 hours and the results revealed that the synthesized nanocomposites were robust without losing any catalytic activity.