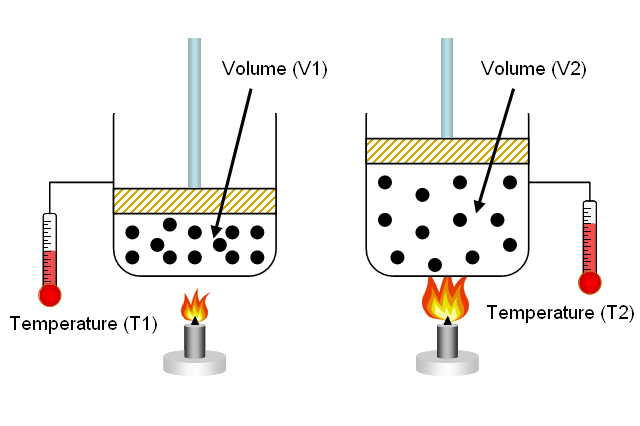
The gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between pressure, volume and temperature of a sample of gas could be obtained which would hold to approximation for all gases. Gases behave in a similar way over a wide variety of conditions because they all have molecules which are widely spaced, and the equation of state for an ideal gas is derived from kinetic theory. The earlier gas laws are now considered as special cases of the ideal gas equation, with one or more variables held constant.
:
Discuss the following laws, referring to their represented equations
Boyle’s Law
Charles’s Law
Avogadro’s Law