Deduce the relation between the reaction rate and substrate concentration for an enzym "HE" in both acidic and basic medium.
Concerning the following:
Kb=[H+][HE]/[H2E+]
Ka=[H+][E-]/[HE]
0[E]
= [HE] + [HES] + [H2E+] + [E-]
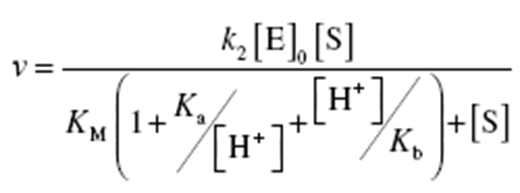
Deduce the relation between the reaction rate and substrate concentration for an enzym "HE" in both acidic and basic medium.
Concerning the following:
Kb=[H+][HE]/[H2E+]
Ka=[H+][E-]/[HE]
0[E]
= [HE] + [HES] + [H2E+] + [E-]