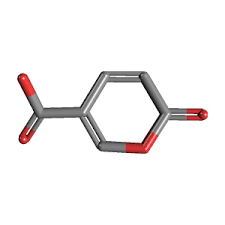
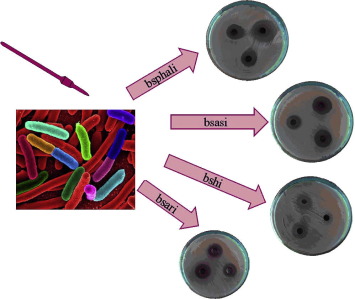
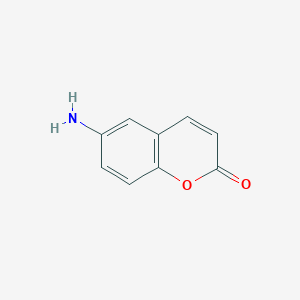
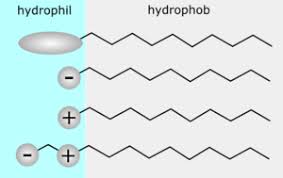
The United Nations in September 2015 agreed on a new global Agenda to take the world on a sustainable pathway. The 2030 Agenda for Sustainable Development involves 17 sustainable development goals (SDGs) for countries to reach by 2030. The sustainable development goals are started to be implemented from January 2016 in all around the world. Implementationof SDGs needs executed policies ...
Read more