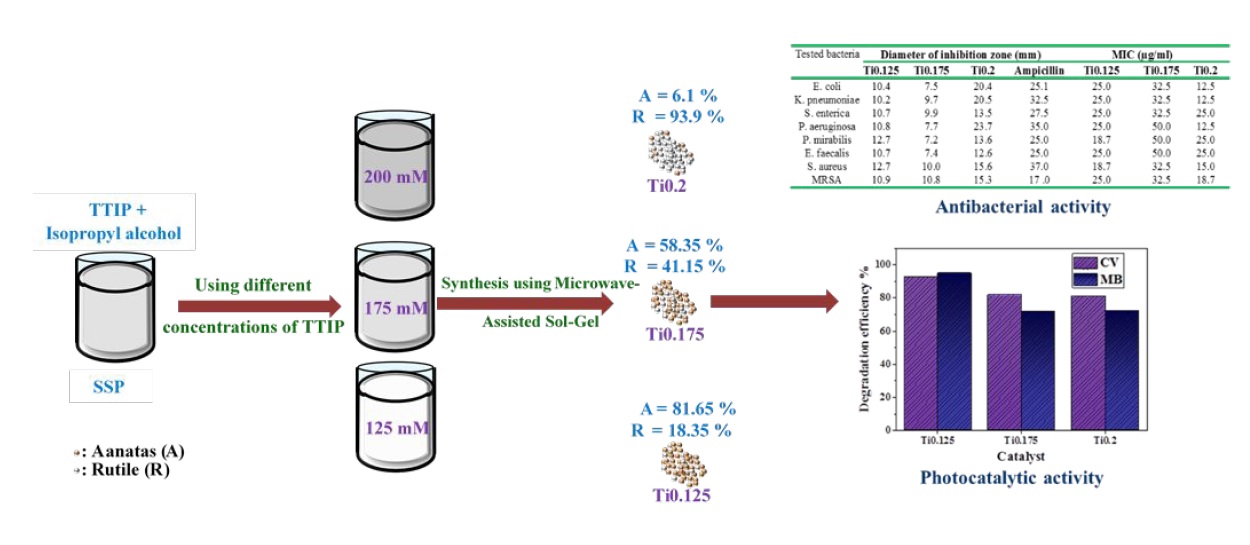
The idea of preparing different ratios of (anatase/rutile) TiO2 mixture has been performed by using microwave assisted sol-gel method and by applying particular concentrations of titanium (IV) isopropoxide as a single source precursor (SSP) with a constant concentration of nitric acid that has been used as structure directing agent. The effect of TiO2 composition ratio of anatase to rutile on physicochemical and photocatalytic properties has been studied. The structural, morphological, and optical properties of the prepared samples have been studied through X-ray diffraction (XRD), high-resolution transmission electron microscope (HRTEM), and UV-Vis Diffuse reflectance spectrum (DRS); respectively. In addition, the chemical group functions have been determined using Fourier transform infrared (FTIR). The characterization results showed that the crystallite sizes were found to be in the range of 30 -70 nm and also revealed the different crystalline phases ratio of anatase and rutile depending on the concentrations of Ti precursor. The photocatalytic degradation of synthesized samples has been evaluated with crystal violet (CV) and methylene blue (MB) solutions under UV light irradiation. The results confirmed that the TiO2 sample containing 81.65 wt% anatase exhibited a high photoactivity on organic dyes degradation. The inhibitory effects of the tested synthesized samples of TiO2 nanoparticles have been determined against different multidrug resistant gram-positive and negative bacterial pathogens. The most active sample was the one with the highest ratio of anatase.