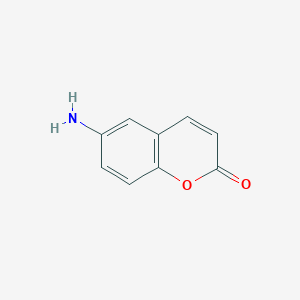
The kinetics of the base hydrolysis of 6-aminocoumarin and some of its derivatives was studied spectrophotometrically in aqueous medium at 298 K under pseudo-firstorder
conditions ([OH-][[[Compound]). The suggested general rate equation can be given as: rate = {k1 ? k2
[OH-]} [complex]. The reasonable mechanism suggested
that the investigated reaction proceeded via the formation of an intermediate and the ring opening of the formed intermediate was the rate determining step. This is the first time in which the kinetics of the formation and decay of the intermediate were followed (at [OH-] B 3.5 9 10-2 mol dm-3).