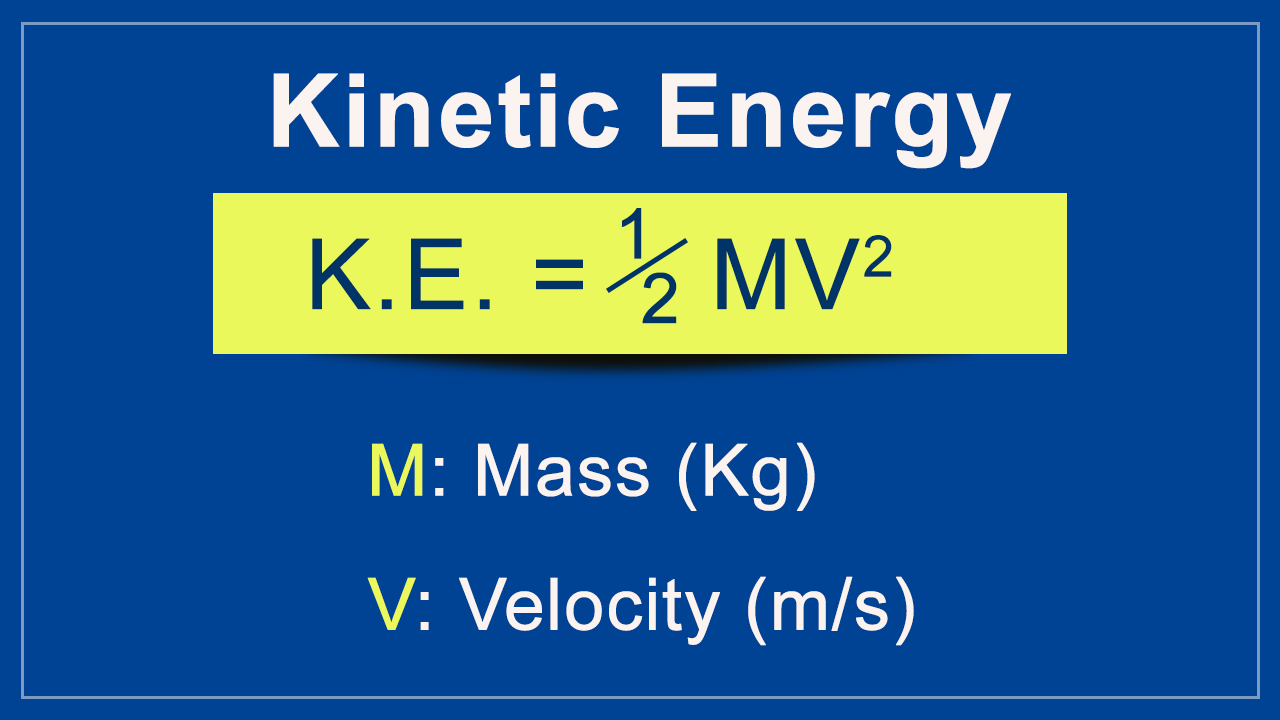
[1]
A tank used for filling helium balloons has a volume of 0.300 m3 and contains 2.00 mol of helium gas at 20.0°C.
Assume that the helium behaves like an ideal gas.
(A) What is the total translational kinetic energy of the gas molecules?
(B) What is the average kinetic energy per molecule?
[2]
What if the temperature is raised from 20.0°C to 40.0°C? Because 40.0 is twice as large as 20.0, is the total translational energy of the molecules of the gas twice as large at the higher temperature?