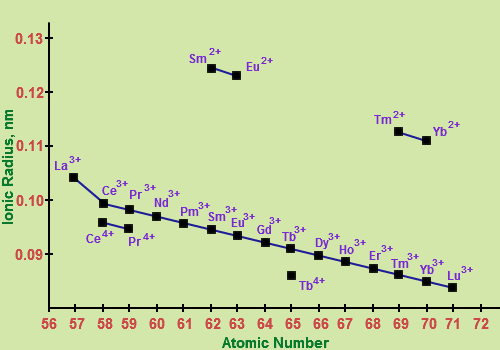
The elements following the lanthanides in the periodic table are influenced by the lanthanide contraction. The radii of the period-6 transition metals are smaller than would be expected if there were no lanthanides, and are in fact very similar to the radii of the period-5 transition metals, since the effect of the additional electron shell is almost entirely offset by the lanthanide contraction.
For example, the atomic radius of the metal zirconium, Zr, (a period-5 transition element) is 159 pm and that of hafnium, Hf, (the corresponding period-6 element) is 156 pm. The ionic radius of Zr4+ is 79 pm and that of Hf4+ is 78 pm. The radii are very similar even though the number of electrons increases from 40 to 72 and the atomic mass increases from 91.22 to 178.49 g/mol. The increase in mass and the unchanged radii lead to a steep increase in density from 6.51 to 13.35 g/cm3.
Illustrate the source of the lanthanide contraction