Competitive inhibition is interruption of a chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for binding or bonding.
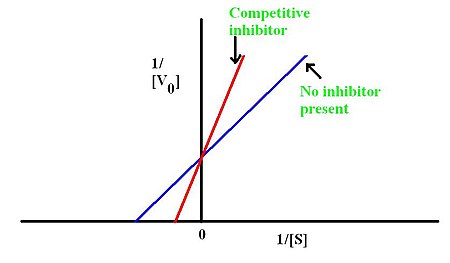
In competitive inhibition of enzyme catalysis, binding of an inhibitor prevents binding of the target molecule of the enzyme, also known as the substrate. This is accomplished by blocking the binding site of the substrate – the active site – by some means. The Vmax indicates the maximum velocity of the reaction, while the Km is the amount of substrate needed to reach half of the Vmax. Km also plays a part in indicating the tendency of the substrate to bind the enzyme. Competitive inhibition can be overcome by adding more substrate to the reaction; therefore, increasing the chances of the enzyme and substrate binding. As a result, this alters only the Km, leaving the Vmax the same. This can be demonstrated using enzyme kinetics plots such as the Michaelis-Menten or the Lineweaver-Burk plot. Once the inhibitor is bound to the enzyme, the slope will be affected, as the Km either increases or decreases from the original Km of the reaction.
Compare between competitive and non-competitive inhibition of the enzymes
.