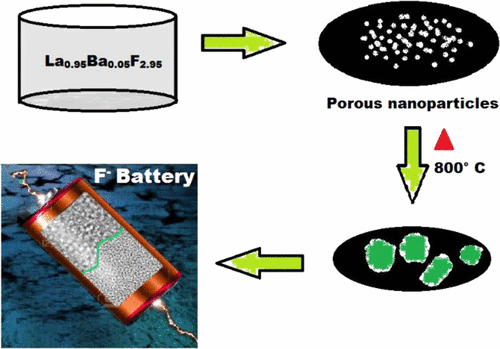
Lithium ion battery is currently the method of choice when it comes to local stationary storage of electrical energy. In the search for an alternative system, fluoride ion battery (FIB) emerges as a candidate due to its high theoretical capacity, and no lithium is needed for its operation. To improve the cycling performance and lower the working temperature of a solid state battery, one of the critical components is the electrolyte which needs advanced performance. This paper aims at developing an electrolyte with enhanced ionic conductivity for fluoride ions, to be used in a FIB. Tysonite La1-xBaxF3-x (0 ≤ x ≤ 0.15) solid solutions were synthesized by a facile wet chemical method, and its ionic conductivity was analyzed using Electrochemical Impedance Spectroscopy (EIS). Composition study shows that the conductivity reaches a maximum of 1.26 × 10-4 S·cm-1 at 60 °C for the La0.95Ba0.05F2.95 pellet sintered at 800 °C for 20 h, which is one order of magnitude higher than the as-prepared pellet and two times higher than the conductivity of sintered ball milled batches. The reason for this dramatic increment is the more efficient decrement of grain boundary resistance upon sintering. Morphological, chemical and structural characterizations of solid electrolytes were studied by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray (EDX) spectroscopy, physisorption by the Brunauer-Emmett- Teller (BET) method and Transmission Electron Microscopy (TEM). Electrochemical testing was carried out for the FIB cell using La0.95Ba0.05F2.95 as electrolyte due to its highest conductivity among the compositions, Ce as anode and BiF3 as a cathode. The cycling performance was found to be considerably improved when compared to our earlier work, which used the ball milled electrolyte.