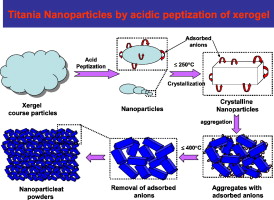
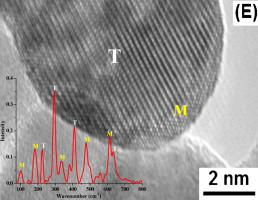
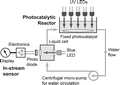
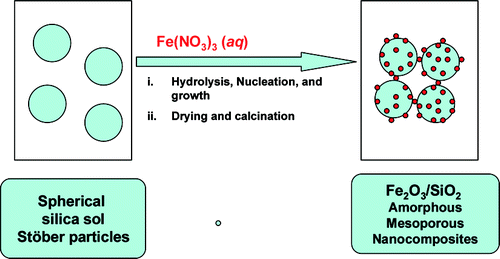
Thermally stable and insoluble silver salts of 12-molybdophosphoric acid with varying amount of Ag cations were prepared. XRD results indicated the presence of single phase of AgxH3xPMo12O40 (0 < x < 3). FTIR and Raman results indicated that Ag was incorporated in the secondary structure of Keggin ion. The catalytic conversion of ethanol increased in the order of H3PMo > ...
Read more