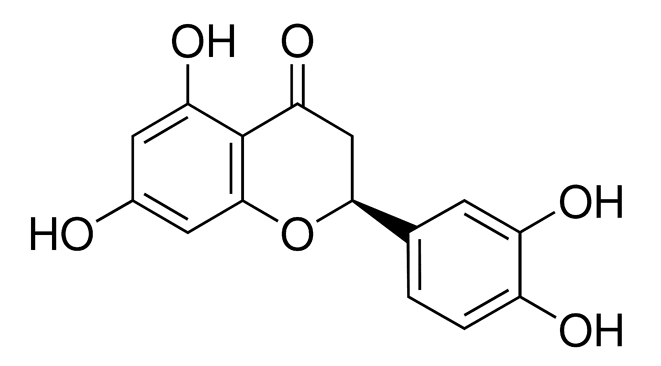
The metabolic oxidation pathways of dietary flavonoid eriodictyol (Er) are not very well-probed. In the present work, the electrochemical oxidation behavior of Er was studied in aqueous Britton-Robinson (B-R) buffer solution using cyclic voltammetry (CV), chronoamperometry (CA), and bulk-electrolysis (BE). The oxidation products and reaction pathways of Er in the absence and the presence of glutathione (GSH) were proposed and identified in view of the results obtained by ultra-highperformance liquid chromatography coupled with mass spectrometry (UPLC-MS). In the absence of GSH, eriodictyol shows one quasi-reversible oxidation process at E1/2=0.305 V, followed by a totally irreversible anodic peak at a more positive potential (Epa=1.05 V vs. Ag/AgCl, 3 M KCl). Putatively, the first process corresponds to the oxidation of the catechol moiety on the B ring of Er while the second one is attributed to the oxidation of the resorcinol moiety on the A ring. In the presence of GSH, however, the anodic oxidation of Er was proposed to be an ECEC-type mechanism. The Er molecule first underwent a two-electron oxidation coupled with loss of two-proton to generate the corresponding quinone, which was either reduced to the original Er molecule by GSH, or further interacted with GSH to produce mono- and biglutathione conjugates of Er. The proposed mechanism was confirmed by digital simulation of the cyclic voltammograms.