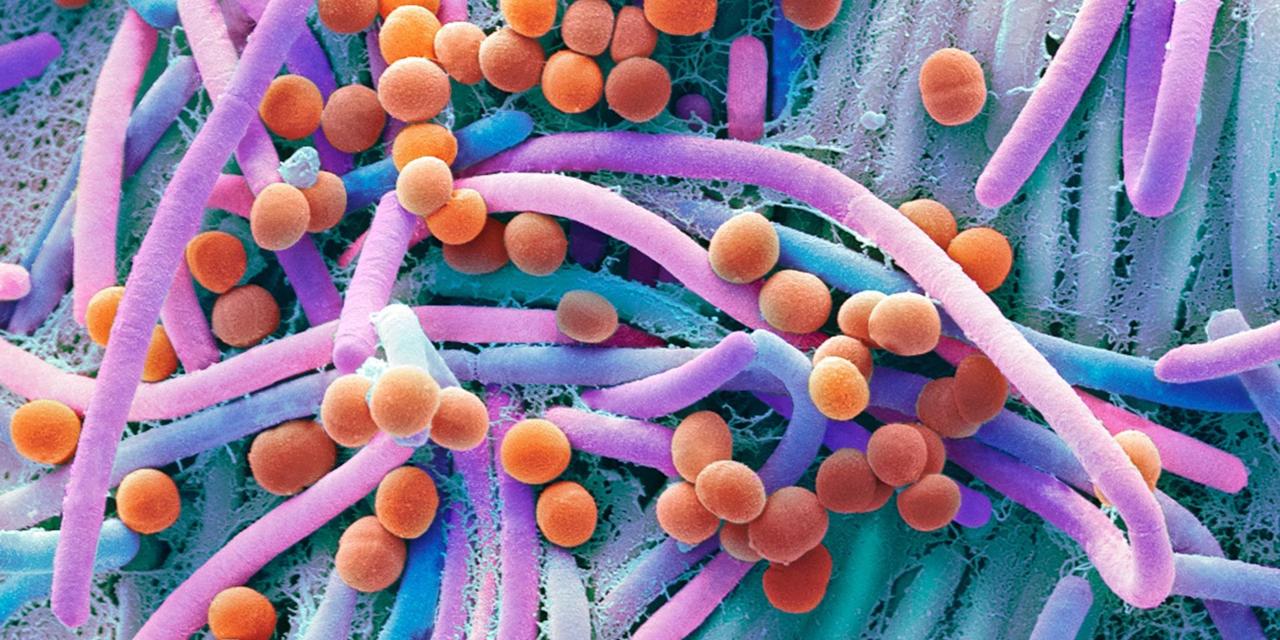
Azomethine amino ligands derived from the condensation of 3-methoxysalicylaldehyde (MS) or 4-diethylaminosalicylaldehyde (DS) with α-amino acids (L-phenylalanine (P) and DL-tryptophan (T)) were synthesized. All ligands were analyzed by IR, 1H NMR, 13C NMR and their melting points. Interaction of the obtained azomethine amino ligands with metal salts produced novel nano sized Fe (II) and Cu (II) complexes. The isolated complexes were characterized by elemental analysis, infrared spectra, ultraviolet-visible and thermal analysis (TGA) in dynamic air atmosphere. The Kinetic and thermal parameters were computed from the thermal data using Coast and Redfern method. The molar conductance values of complexes are relatively low, indicating the non-electrolytic nature of the complexes. Magnetic susceptibility measurements show that the investigated complexes are paramagnetic. Moreover, the stability constants of the prepared complexes were determined spectrophotometrically. The analytical results suggest that amino acid Schiff bases behave as dibasic tridentate ONO ligands, and coordinate with Fe (II) and Cu (II) ions in octahedral geometry according to the general formula [M (HL) 2]. nH2O. The particle size of the prepared complexes was determined using TEM and it was found to be in nano scale. Moreover, the antimicrobial effects of the ligands and their complexes were screened against some types of bacteria such as Bacillus subtilis (+ ve), Escherichia coli (-ve) and Micrococcusluteus (+ ve) and other types of fungi such as Asperagillus niger, Candida glabrata and Saccharomyces cerevisiae. The results of these studies …