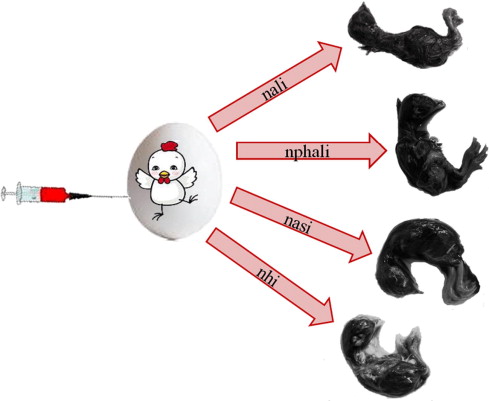
New Fe(II) Schiff base amino acid complexes derived from the condensation of o-hydroxynaphthaldehyde with l-alanine, l-phenylalanine, l-aspartic acid, l-histidine and l-arginine were synthesized and characterized by elemental analysis, IR, electronic spectra, and conductance measurements. The stoichiometry and the stability constants of the complexes were determined spectrophotometrically. The investigated Schiff bases exhibited tridentate coordination mode with the general formulae [Fe(HL)2]·nH2O for all amino acids except l-histidine. But in case of l-histidine, the ligand acts as tetradentate ([FeL(H2O)2]·2H2O), where HL = mono anion and l = dianion of the ligand. The structure of the prepared complexes is suggested to be octahedral. The prepared complexes were tested for their toxicity on chick embryos and found to be safe until a concentration of 100 μg/egg with full embryos formation. The interaction between CT-DNA and the investigated complexes were followed by spectrophotometry and viscosity measurements. It was found that, the prepared complexes bind to DNA via classical intercalative mode and showed a different DNA cleavage activity with the sequence: nhi > nari > nali > nasi > nphali. The thermodynamic Profile of the binding of nphali complex and CT-DNA was constructed by analyzing the experimental data of absorption titration and UV melting studies with the McGhee equation, van’t Hoff’s equation, and the Gibbs–Helmholtz equation.