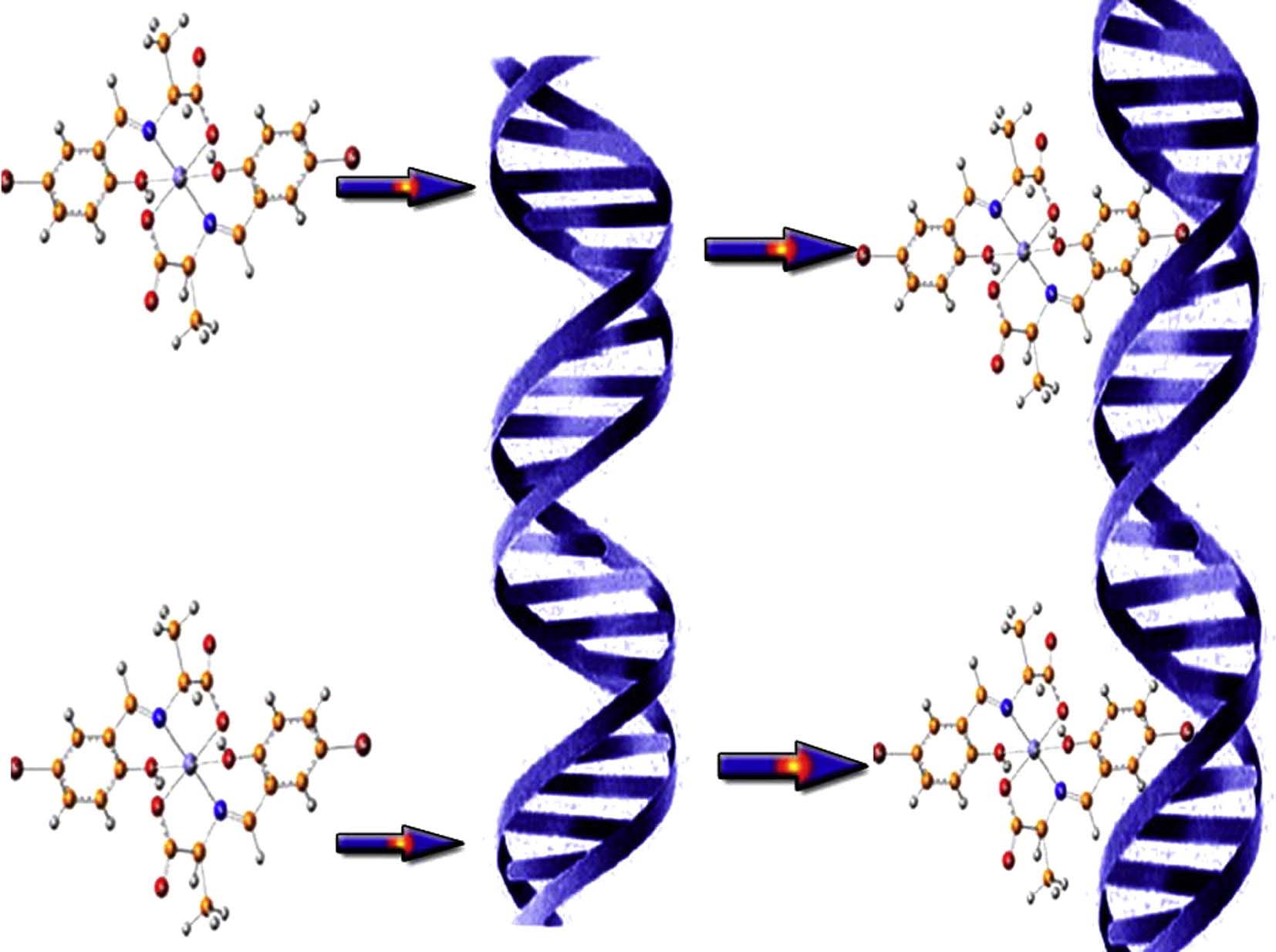
New series of Fe(II) complexes and Schiff base amino acids have been designed and synthesized from the bioactive ligands by condensation of 5-bromo-2-hydroxybenzaldehyde and α-amino acids (l-alanine (ala), l-phenylalanine (phala), l-aspartic acid (aspa), l-histidine (his) and l-arginine (arg)). Elemental analyses, infrared, ultraviolet–visible spectra, as well as conductivity and magnetic susceptibility measurements are used to elucidate the structure of the newly prepared iron(II) complexes. Moreover, the stoichiometry and the stability constants of the prepared complexes have been determined spectrophotometrically. The results suggest that the prepared Schiff base amino acid ligands behave as dibasic tridentate ONO ligands and bind to Fe(II) in octahedral geometry according to the general formula [Fe(bs:aa)2].nH2O. The DNA interaction of these complexes was tested at pH = 7.2, by using electronic absorption spectra and viscosity measurements. The experimental results indicated that the investigated complexes could bind to DNA via intercalative mode and showed a different DNA binding activity according to the sequence: bsari > bshi > bsali > bsasi > bsphali. Moreover, the prepared compounds are tested for their in vitro antibacterial activity against three types of bacteria, Escherichia coli, Pseudomonas aeruginosa and Bacillus cereus. The results show that the metal complexes are more reactive with respect to their corresponding Schiff base ligands.