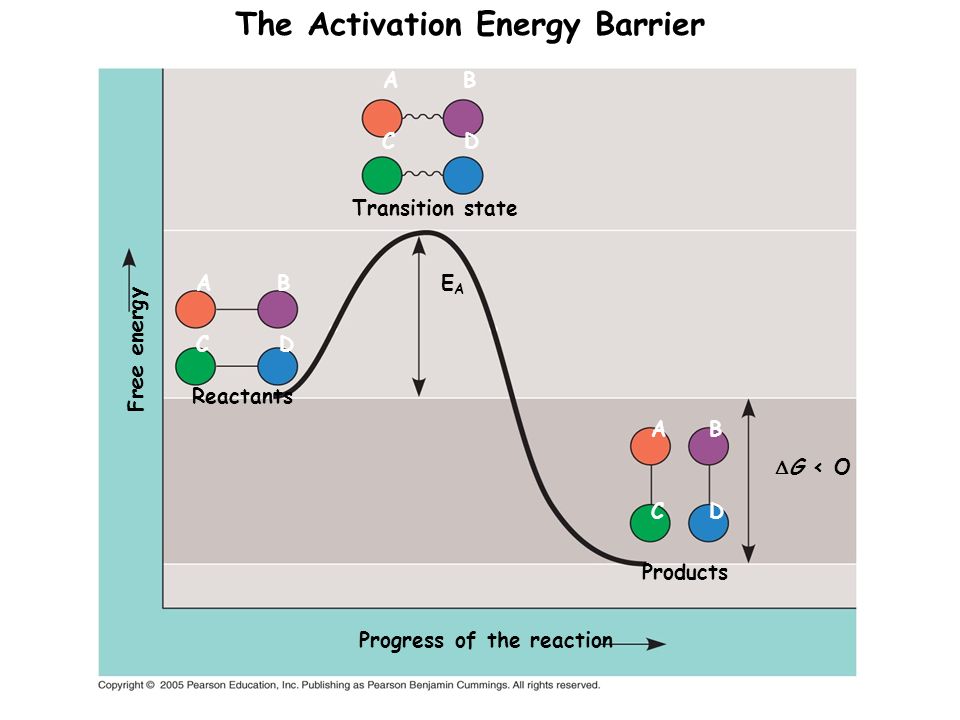
Base-catalyzed hydrolysis of 6-nitro-2H-chromen-2-one (NC) and 6-nitro-2H-chromen-2-one-3-carboxylic acid (NCC) in binary water/methanol and water/acetone mixtures were studied kinetically at 298 K. The changes in the activation barrier of the investigated compounds from water to water/methanol and water/acetone mixtures were estimated from the kinetic data. Solvent effects on the reactivity trends were analyzed into initial state and transition state components. These were determined from the transfer chemical potentials of the reactants and the kinetic data. The transfer chemical potentials δmμθ for NC were derived from its solubilities in water/methanol and water/acetone mixtures, and the transfer chemical potentials for NCC− anion were derived from solubility data for its calcium, cerium and lanthanum salts. Base-catalyzed hydrolysis of NC and NCC in water/methanol and water/acetone mixtures follow a rate law with kobs=k2·c(HO−) and kobs=k1+k2·c(HO−), respectively. The decrease in the rate constants of base-catalyzed hydrolysis of NC as methanol% or acetone% increases is dominated by the initial state (IS), while the decrease in the rate constants of base-catalyzed hydrolysis of NCC as methanol% or acetone% increases is dominated by the transition state (TS).