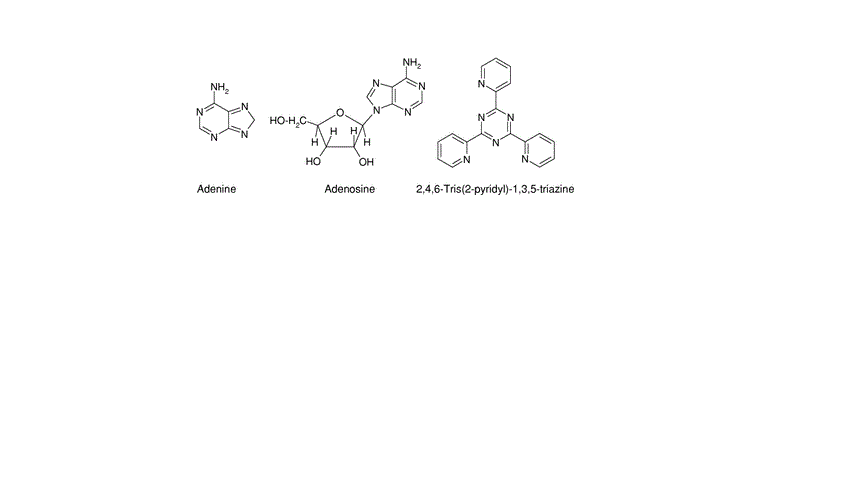
The effect of adenine, adenosine, and 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ) on the electrochemical and corrosion behavior of tin, indium, and tin-indium alloys in 0.5 M HClO4 solution at different temperatures was studied. The inhibition efficiency increases with an increase in the concentration of adenine and adenosine in the case of tin and indium. However, the effect of two mentioned compounds on the corrosion rate of the studied alloys gives an opposite effect. In the presence of TPTZ, the inhibition efficiency increases as the concentration of the inhibitor is increased in the case of tin. In the case of both indium and its investigated alloys, the maximum inhibition efficiency is obtained at the lowest concentration of TPTZ (10−6 M). The adsorption of the studied compounds is found to obey the Frumkin adsorption isotherm. The standard enthalpy ΔH∘ads,entropy ΔS∘ads, and free energy changes of adsorption ΔG∘ads are calculated and discussed.